| Infos
Properties
Formation
Occurence
Mining
Fashioning
Grading
Historic
Diamonds
Introduction
Koh-i-Noor
Blue Hope
Cullinan
Shah
Great Mogul
Florentine
Sancy
Regent
Overview
Courses
Use of diamond


Copyright
by
Maisenbacher Diamonds
B.V.B.A.
B-2018
Antwerpen
| |
The
hard facts are here:
Diamonds in figuresDiamond is pure, crystalline carbon. But
it differs greatly from the examples of carbon we know better:
e.g. graphite, the black stuff in pencils.From this it differs
fundamentally in its orderly crystal form. Uncut diamonds form
mainly octohedrons and dodecahedrons, but also cubes and more
complex forms (24 or 48 faces) as well as crystal combinations.Through
its extraordinary formation under great heat and great pressure,
it has many extraordinary properties.
Known to many is its enormous hardness, the technical term is
Hardness 10 on the Mohs hardness scale, hence the maximum value.
Which means that no other known substance can "scratch" a diamond.
Here we talk about the "scratch" hardness. But there is also
the "polishing " hardness.
In this domain the diamond is again without equal : It is about
140 times as hard as the next harder gemstones - ruby and sapphire.
That is why it is perceived as "forever". Immediately the question
arises how in fact a natural diamond is dressed. After all,
we value it on account of its sparkle, its fire, its brilliance,
and these arise only through the human hand (natural reflection
of a diamond : 17%). In this connection a dictum by G. F. Herbert
Smith appears quite remarkable : "A rough diamond [...] is no
more attractive to the eye than a piece of washing-soda." ...
So how is it done?
The answer is: "with diamonds", and, indeed, it is only possible
with the aid of diamonds or diamond powder (on polishing wheels)
to dress a diamond. That is why in earlier times diamonds were
not at all or only a little dressed (polished) - in most cases
merely to remove minor flaws.
In India it was even forbidden to dress diamonds. Only in recent
times (roughly since 1456) have diamonds been dressed approximately
the way we know today. This phenomenon stems from the hardness
differing in various directions (professionally speaking: "hardness
anisotropy"). And this again is because of the arrangement of
the atoms in the diamond, which differs in various directions;
also the intervals between the atoms and their bonding powers
differ.
But this enormous hardness also has a big disadvantage: The
diamond is rather brittle. Even a light blow can destroy it.
In the past this property was utilised when a large natural
diamond was to be cut into smaller ones (Historic Diamonds /
Cullinan). A light (sometimes historic) blow - and you had several
small ones.
The specialist talks about "perfect cleavability" here. Today,
the diamond is sawn - with a diamond-coated circular blade. |
|
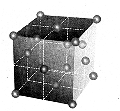
18
carbon atoms form the basic building block of the diamond
|

these,
e.g., constitute the . octohedron form
|
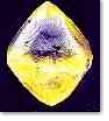
and this
is a piece of "washing soda" - a rough diamond |
| |

|


